When Military Might Relied on Urine
A look at the chemistry behind the historical production of saltpeter from urine
Gunpowder has come to dominate battlefields since the 15th century (1400 to 1500). It is an explosive mixture of potassium nitrate (KNO₃), charcoal (C) and sulfur (S).
Charcoal and sulfur are relatively easy to obtain. The challenge is obtaining potassium nitrate, also known as saltpeter and niter, which historically made potassium nitrate an extremely important strategical resource. That is why this story will be all about potassium nitrate. There are many accounts of historical saltpeter production. However, each time I read one of these accounts I was left scratching my head because the whole process seemed so utterly bizarre. I wrote this story for those, who like me, are curious about the science behind historical saltpeter production.
The historical difficulty of obtaining saltpeter (potassium nitrate) helps explain why China was the first country to develop gunpowder. In Europe, there are no natural deposits of saltpeter, while in China, it has historically been an abundant enough for the Arabs to refer to saltpeter as "Chinese snow." You can understand why if you look at what naturally occurring saltpeter looks like. The ground on the picture below is not covered in snow, but saltpeter.

We are going to look at different ways of obtaining saltpeter. Specifically, why urine and manure played such a central role in the production of saltpeter.
When I started studying this subject, I found it rather bewildering as there are so many approaches and techniques. It helps to observe that formation and production of saltpeter is strongly tied to the nitrogen cycle.
The Nitrogen Cycle
Saltpeter is really all about nitrogen, just like gunpowder is all about saltpeter. Nitrogen is the difficult ingredient to get hold of. Thus, to understand the formation of saltpeter, it helps to understand the nitrogen cycle.
All nitrogen originates from our atmosphere as N₂ gas. Humans and plants cannot utilize this gas directly. Bacteria and plants are primarily responsible for stuffing nitrogen atoms into more complex chemical compounds.
You can see from the diagram that the soil contains bacteria, which can convert ammonia (NH₃) to nitrate (NO₃). Once you have nitrate, most of the job is done because it can be combined with potash to create saltpeter (KNO₃). Potash is a collective term for various compounds containing potassium (K), such as potassium carbonate (K₂CO₃).
With the nitrogen cycle in mind, we can look at how saltpeter was made in the European Renaissance.
Making Saltpeter in the Renaissance
Whether making saltpeter, iron, petroleum, or alcohol, the key thing that we are doing is finding ways of separating the desired elements from the undesirable ones. When we learn about chemistry in school, we tend to think of chemistry as being about combining cool chemicals to get fun effects. In reality, chemistry is just as much about separation as combination of chemical compounds. The Dutch word for chemistry is "scheikunde", which means art of separation.
Different compounds are separated in different ways. You can separate alcohol from water by boiling the water. You can separate a mix of sand and salt by adding water, which causes the salt to be dissolved in water but not the sand. Sand will accumulate at the bottom, and you can pour out the water. Later, if you let water evaporate you are left with salt. This is how regular sodium chloride salt (NaCl) is produced. Seawater is made to evaporate.
Saltpeter (potassium nitrate) is extracted similarly. It dissolves in hot water, so you can use that property to separate it from other things. On Wikipedia, we can find various methods. One is to get it from mineral sources:
In this book, al-Rammah describes first the purification of barud (crude saltpeter mineral) by boiling it with minimal water and using only the hot solution, then the use of potassium carbonate (in the form of wood ashes) to remove calcium and magnesium by precipitation of their carbonates from this solution, leaving a solution of purified potassium nitrate, which could then be dried.
You can get it from caves where bats or birds have deposited excrements called guano.
Extraction is accomplished by immersing the guano in water for a day, filtering, and harvesting the crystals in the filtered water.
In Europe, saltpeter was historically produced in a nitrary, also known as a saltpeter works. At its most fundamental level, a nitrary is about extracting nitrogen from urine and converting it to potassium nitrate (KNO₃). The nitrogen is bound in a compound called urea (NH₂)2CO, which is found in urine. A nitrary is about utilizing numerous bacteria found in animal dung and dirt to perform conversion of urea to potassium nitrate (saltpeter).
Let me phrase this in another way: Urine is the feedstock to this process. The bacteria-rich soil and manure just offer the machinery to covert the urine to saltpeter. It is a very slow process that takes many months.
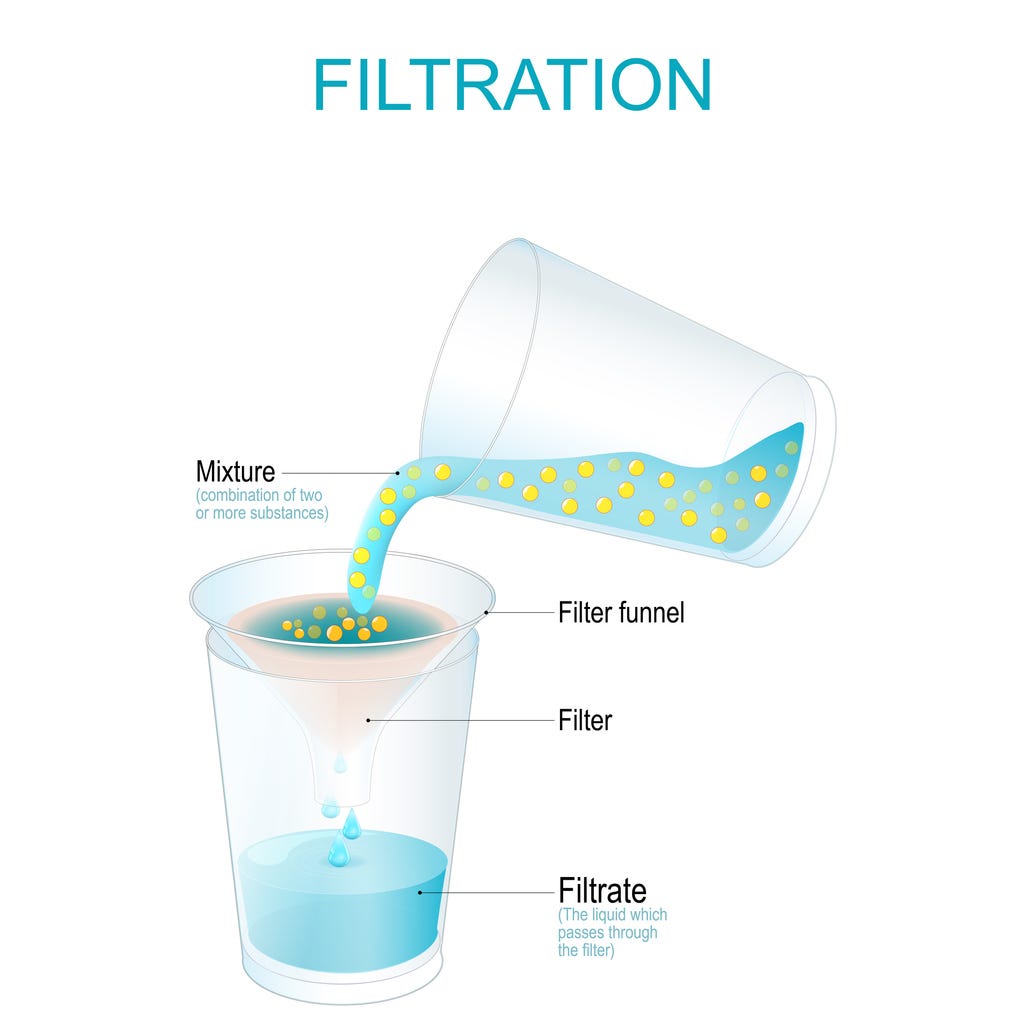
Just as you can separate sand from salt by dissolving salt in water, you put the piles of dirt and dung into buckets of water to dissolve saltpeter and other salts found in the dirt. You can extract the water to get the salts and saltpeter separated from the dirt.
You will recognize several of the bacteria from the nitrogen cycle. The first step in the process is bacteria containing the enzyme urease , which break down urea to ammonia.
Other bacteria in the dung and dirt convert ammonia (NH₃) to nitrate (NO₃). Usually, potash is added to the mix to supply the potassium we need to bind to the nitrate to form potassium nitrate (KNO₃).
You may think this is mission accomplished, but these kinds of processes tend to be complex. When I said chemistry is all about separation, I wasn't joking. Typically, you start with numerous undesirable elements. Each separation process only gets rid of a couple of the elements you don't want.
The brown, dirty water that you leached out of the dung and dirt still contains plenty of things we don't want. Many of these compounds are what we call nitrates. A nitrate is just a chemical compound with a NO₃ attached to it. Saltpeter (KNO₃) is an example of a nitrate.
Keep reading with a 7-day free trial
Subscribe to Erik Examines to keep reading this post and get 7 days of free access to the full post archives.